To read the original article in full go to : CRISPR: A FutureFactual Deep Dive.
Below is a short summary and detailed review of this article written by FutureFactual:
CRISPR: A FutureFactual Deep Dive
FutureFactual Deep Dives take you behind the story, into the science behind the headlines. Handpicked and verified by the FutureFactual team, Deep Dives bring you the sharpest, most essential content to get you fully up to speed, whatever the topic.
Here, we’ve gathered the most insightful videos, podcasts, and articles from trusted voices. Together, they’ll bring you up to speed on one of the most transformative breakthroughs in modern biology: CRISPR gene-editing. Whether you’re new to the subject or ready to dive into the cutting edge of genome engineering, these are the pieces worth your time.
THE STORY SO FAR…
The future is editable, and CRISPR is holding the pen.
But what exactly is CRISPR? And how about the headline grabbing ‘CRISPR-Cas9’? How did it go from a strange pattern in bacterial DNA to one of the most powerful technologies ever invented? And what will it mean if we can’t control, or fail to fully harness, its potential?
Here’s the lowdown…
WHAT IS CRISPR?
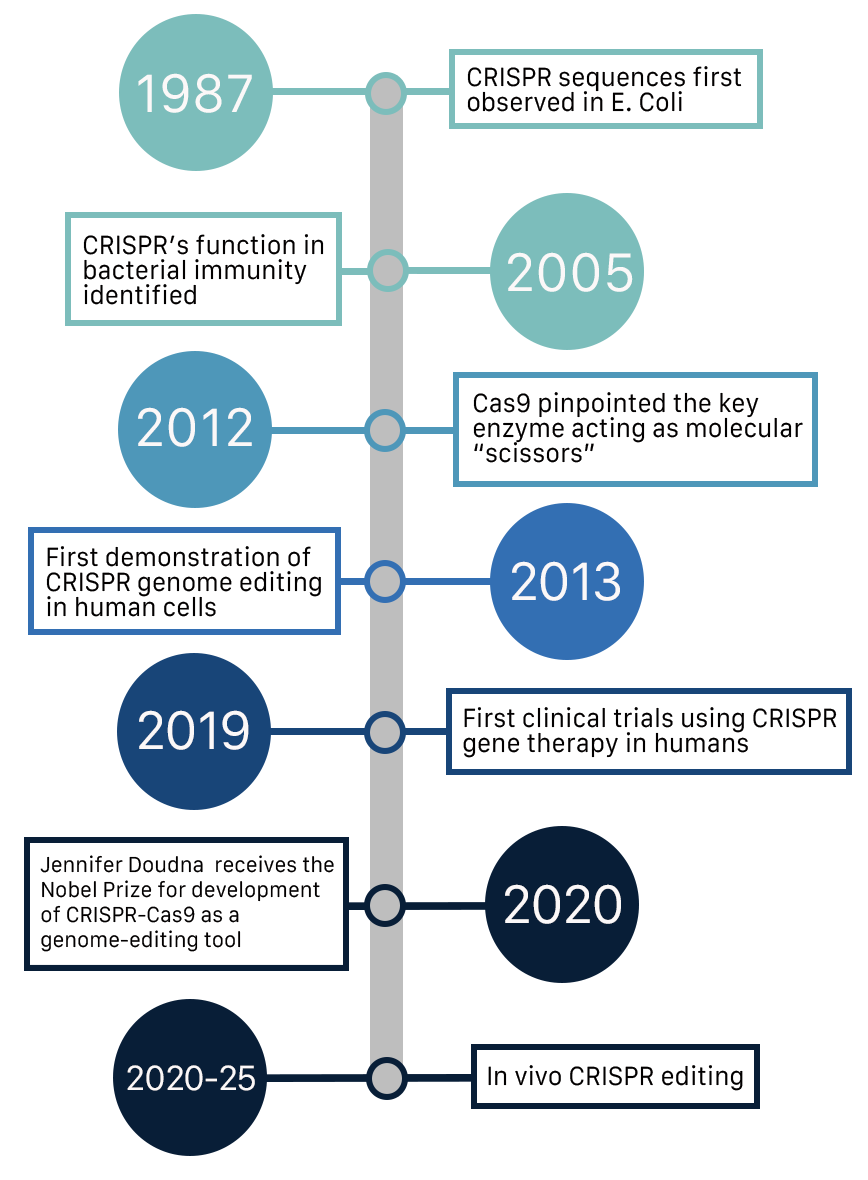
CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats. Quite the mouthful! Very simply, its the overall system that bacteria use to recognize and defend against viruses. It includes stored “memory” of past viral attackers and the machinery to find matching DNA.
And CRISPR-Cas9 is one specific version of that system. In this version, Cas9 is an enzyme which acts like a pair of molecular scissors - in other words, its the protein that actually cuts DNA.
SO WHAT’S THE BIG DEAL?
Scientists realised they could repurpose this natural defence system into a programmable gene-editing tool: change the guide RNA, and Cas9 can cut almost any DNA sequence.
This allows scientists to guide Cas9 to a chosen DNA sequence so it can cut and allow genes to be changed. This has huge implications for health, offering the potential to cure genetic diseases like sickle cell, muscular dystrophy, and certain forms of blindness. Beyond human health, it could also improve agriculture by creating disease- and drought-resistant crops, and it can help combat climate change by modifying microbial communities to reduce greenhouse-gas emissions. Its not an overstatement to say that this technology could transform medicine, food, and the environment.
This clear, animated explainer of how CRISPR–Cas9 works is a few years old now but still offers the perfect grounding in the basics of gene editing if you’re new to the topic.
HOW ARE TREATMENTS DELIVERED?
One of the biggest challenges in CRISPR therapy is delivery — how to get gene-editing molecules safely into the right cells.
Researchers are exploring unexpected vehicles: nanoparticles, engineered viruses, lipid droplets, even biomaterials inspired by everyday substances. Early studies suggest certain sugar-based polymers and biodegradable materials can shield CRISPR components and help them reach their targets more efficiently, offering a surprisingly low-cost and biocompatible toolkit for future therapies.
The field is wide open and full of creativity.
This insightful video offers a look into the details of how CRISPR gene editing therapies are delivered in treatment for Sickle Cell disease.
WHAT COULD CRISPR TECHNOLOGIES DO FOR US?
CRISPR is rewriting the rules of what’s possible with DNA. From life-saving medicine to climate-smart farming and green industry, CRISPR isn’t just science, it’s a toolkit for transforming the world.
As Sci-Show covers in their brilliant episode on this topic, already, CRISPR-based therapies help treat blood disorders, and researchers are exploring its use to improve organ transplants by making animal organs more compatible with humans.
In cancer treatment, CRISPR can target tumor-promoting genes, enhance immune responses, or deliver cancer-fighting agents directly to tumors, with several therapies now in clinical trials.
Beyond medicine, CRISPR could address the climate crisis by creating crops resilient to drought, poor soil, or extreme conditions, while also enhancing taste and nutrition. In industrial applications, it could optimize microbes for ethanol production or biodegradable plastics, improving efficiency and sustainability. While many of these innovations are still experimental or limited by regulation, CRISPR’s precision and flexibility make it a transformative tool with far-reaching possibilities for health, food security, and environmental solutions.
WHAT ARE THE ETHICAL CONCERNS?
CRISPR isn’t just a scientific tool, it’s a societal choice. How do we balance innovation with caution? What responsibility comes with the power to rewrite life? Who decides what’s acceptable to change? And how do we ensure transparency, accountability, and public trust? The Naked Scientist podcast does a brilliant job of unpacking these tricky questions.
CRISPR ignites fierce ethical debates. While early headlines promised flawless gene editing, reality is more complicated: off-target edits, unexpected cellular responses, and delivery challenges in complex tissues can make outcomes unpredictable. Some researchers suggest alternative tools, like TALENs, zinc-finger nucleases, or emerging RNA-based editors, may outperform CRISPR in certain contexts. Others argue the biggest hurdle isn’t the tool at all, it’s biology itself. Genes rarely act alone, and editing one can ripple across entire networks, highlighting the need to understand systems-level interactions before intervention.
The debate becomes even more charged when germline editing, changes to embryos, sperm, or eggs, is considered. Unlike somatic editing, germline changes are heritable, affecting future generations. Advocates see the potential to eliminate devastating genetic diseases; critics warn of unforeseen consequences and moral dilemmas. The 2018 birth of CRISPR-edited babies sparked global outrage and calls for regulation, and today most countries ban or strictly limit germline edits.
Beyond humans, CRISPR could reshape ecosystems through gene drives, controlling pests or disease vectors but mistakes could cascade uncontrollably. Experts urge caution: the power to rewrite life is profound, and society must weigh innovation against responsibility, transparency, and ethical oversight. The question remains: just because we can, should we?
CRISPR BIOHACKERS
Now, a bold new frontier is emerging: DIY CRISPR biohackers, people experimenting on themselves with gene-editing kits in garages and home labs. Driven by curiosity, and sometimes desperation, these pioneers tinker with their own DNA, testing everything from immune tweaks to potential disease resistance.
Overthink’s episode on this phenomenon delves deep into the questions underpinning this risky practice and interviews one of the pioneers of the movement.
The motivation isn’t just thrill-seeking; many argue that CRISPR’s power could deepen global inequalities. If cutting-edge gene therapies remain confined to wealthy nations or elite clinics, the gap between rich and poor in health and longevity could widen dramatically. Self-experimentation becomes, in their eyes, a radical way to claim access to tools they believe should belong to everyone. But the stakes are high: unintended edits could be harmful or irreversible. Advocates say the solution lies in equitable access, global regulation, and open-science initiatives that make gene therapies safer and available across borders. Biohacking sparks both excitement and urgent ethical questions about fairness, safety, and the future of human enhancement.
THE NEXT CHAPTER IN CRISPR
Combining CRISPR with metagenomics enables a new field: precision microbiome editing, allowing us to adapt specific microbes within complex communities. This approach could correct dysfunctional human microbiomes linked to asthma, obesity, diabetes and Alzheimer’s.
As covered in this brilliant Ted Talk from Microbiologist, Jennifer Doudna, specific livestock microbiomes can naturally reduce methane, a major driver of climate change, by up to 80%, but current interventions are costly and temporary. Precision microbiome editing could modify an animal’s microbiome early in life, cutting methane emissions for its entire lifetime and improving feed efficiency. Future applications could target methane emissions from rice paddies, landfills, and wastewater, addressing sources responsible for up to two-thirds of global methane output.
By editing the very microbes that inspired CRISPR, we can collaborate with nature to improve global health and environmental resilience.
CONCLUSION
We are entering the most decisive era yet for gene editing. New CRISPR variants, advanced delivery strategies, and rapidly expanding clinical trials are pushing the boundary of what’s possible. The next ten years could reveal whether CRISPR technologies become routine in therapies for genetic disease, a backbone of sustainable agriculture, a tool for controlling global health threats or whether ethical, financial or regulatory constraints require us to redefine our ambitions.
Whatever the outcome, the coming decade promises discoveries that will reshape how we understand and shape life itself.